Vet practice & suppliers
For vets, vet nurses, practice staff, suitable qualified persons, wholesalers, retailers and dispensers
Monthly Medicines Update
Get the latest information about new marketing authorisations and changes to existing authorisations that relate to the safe use of the product.

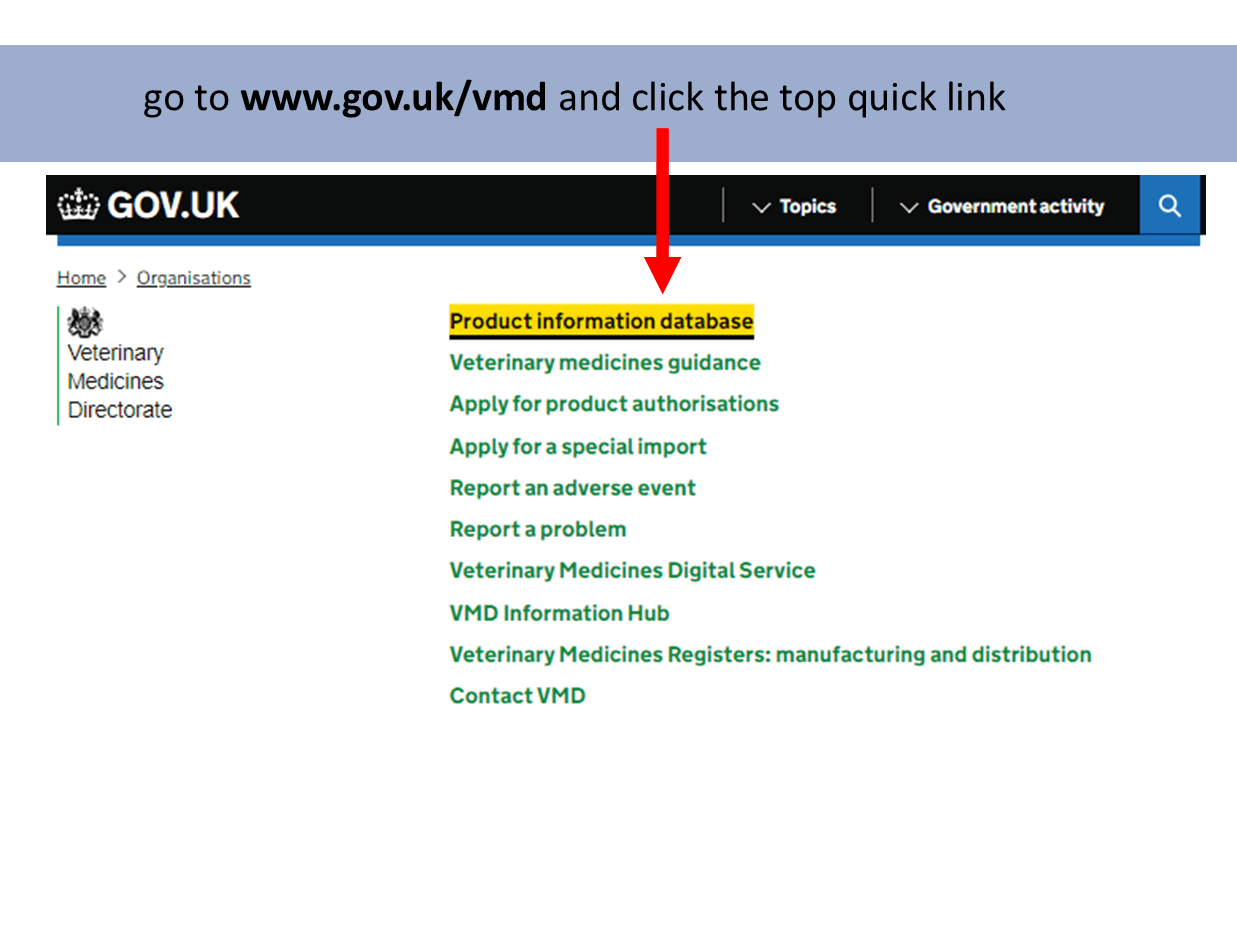
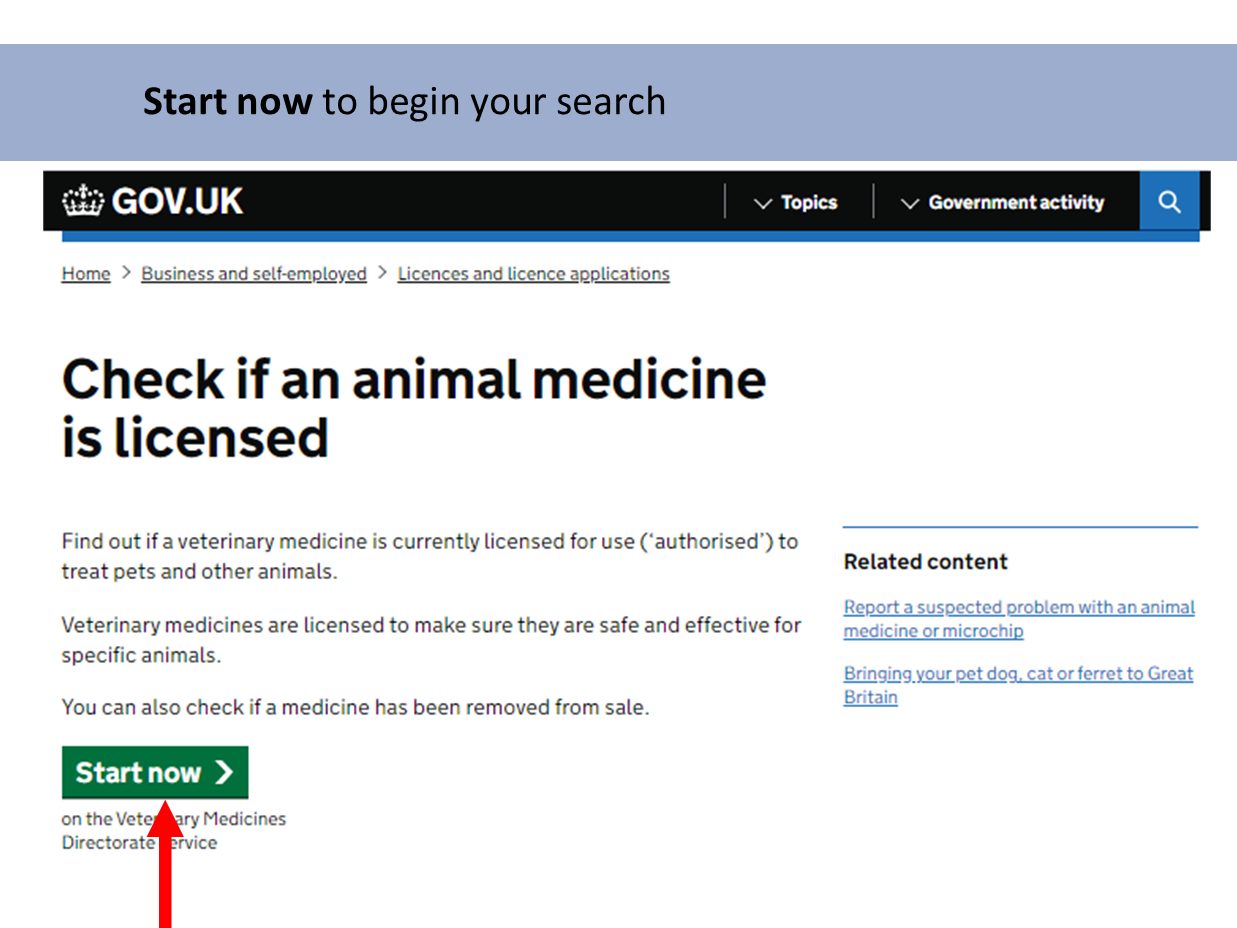
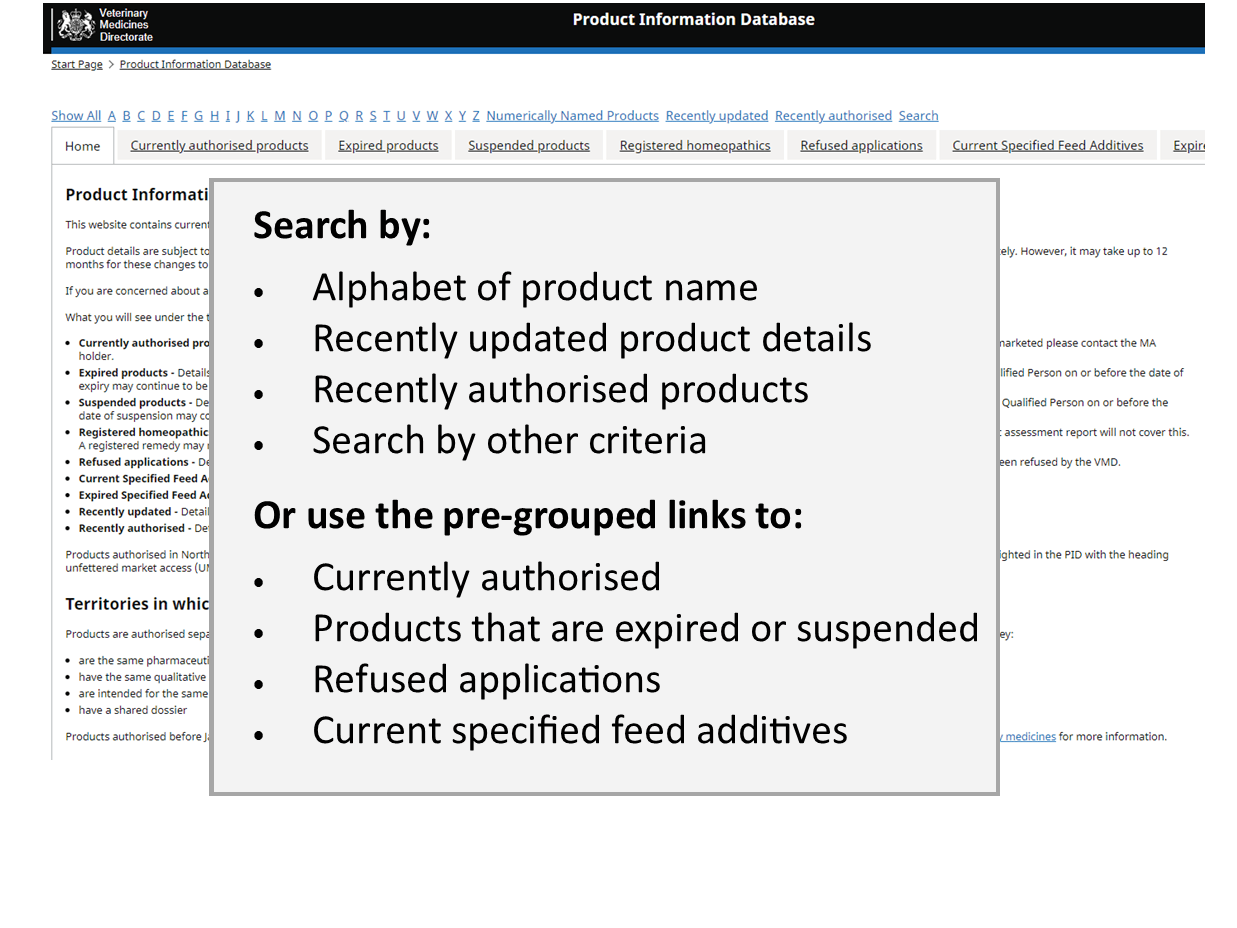
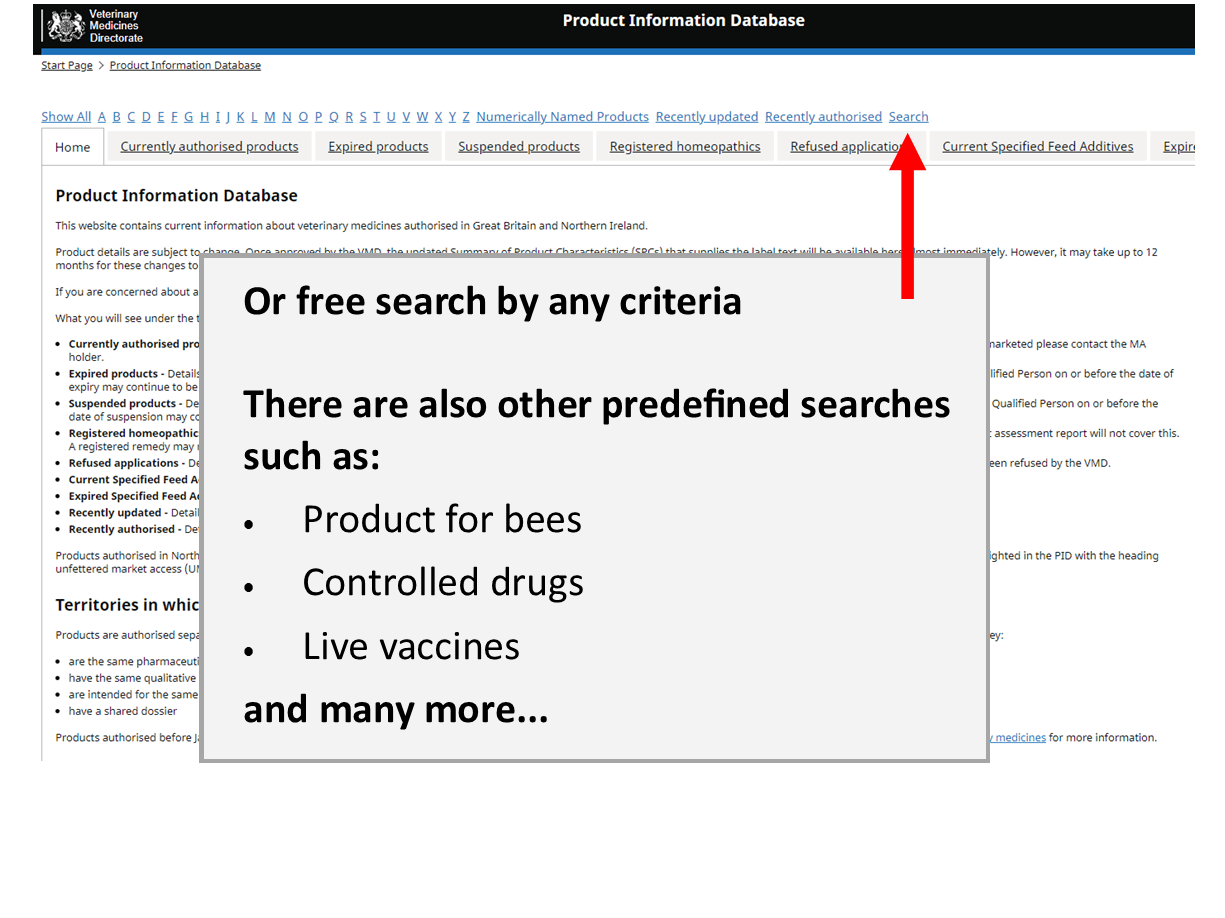
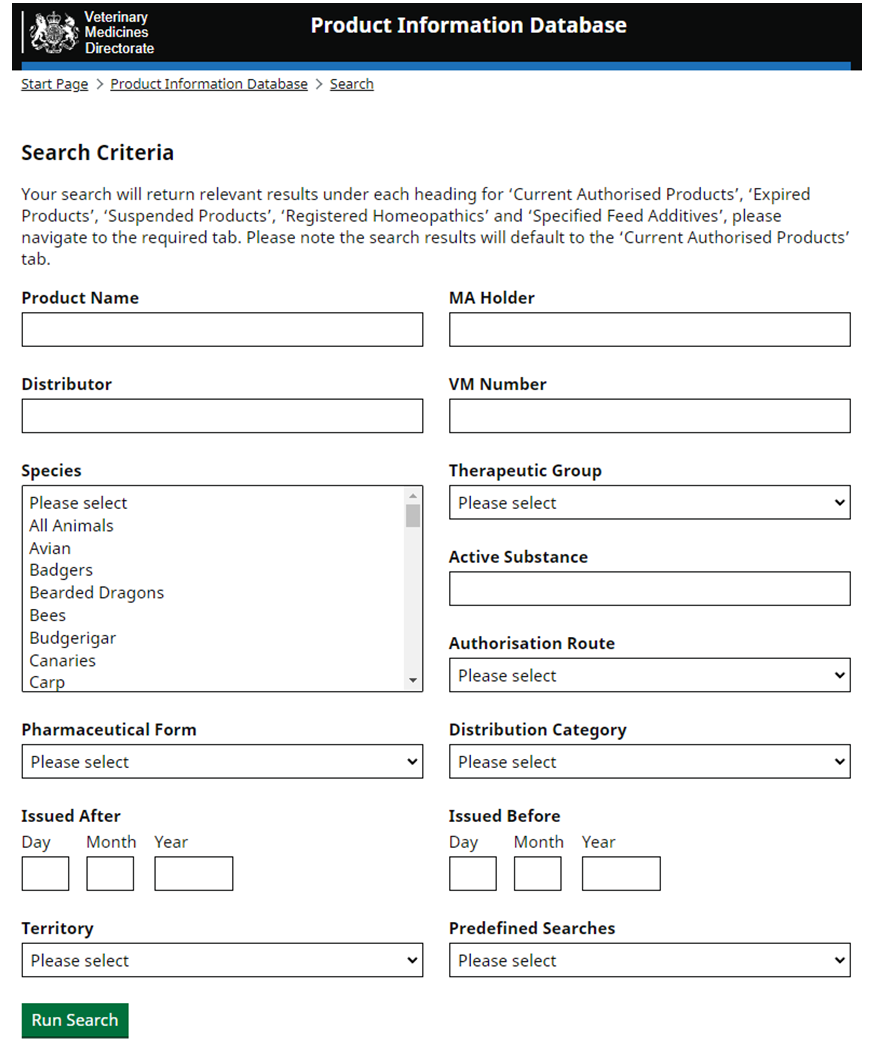
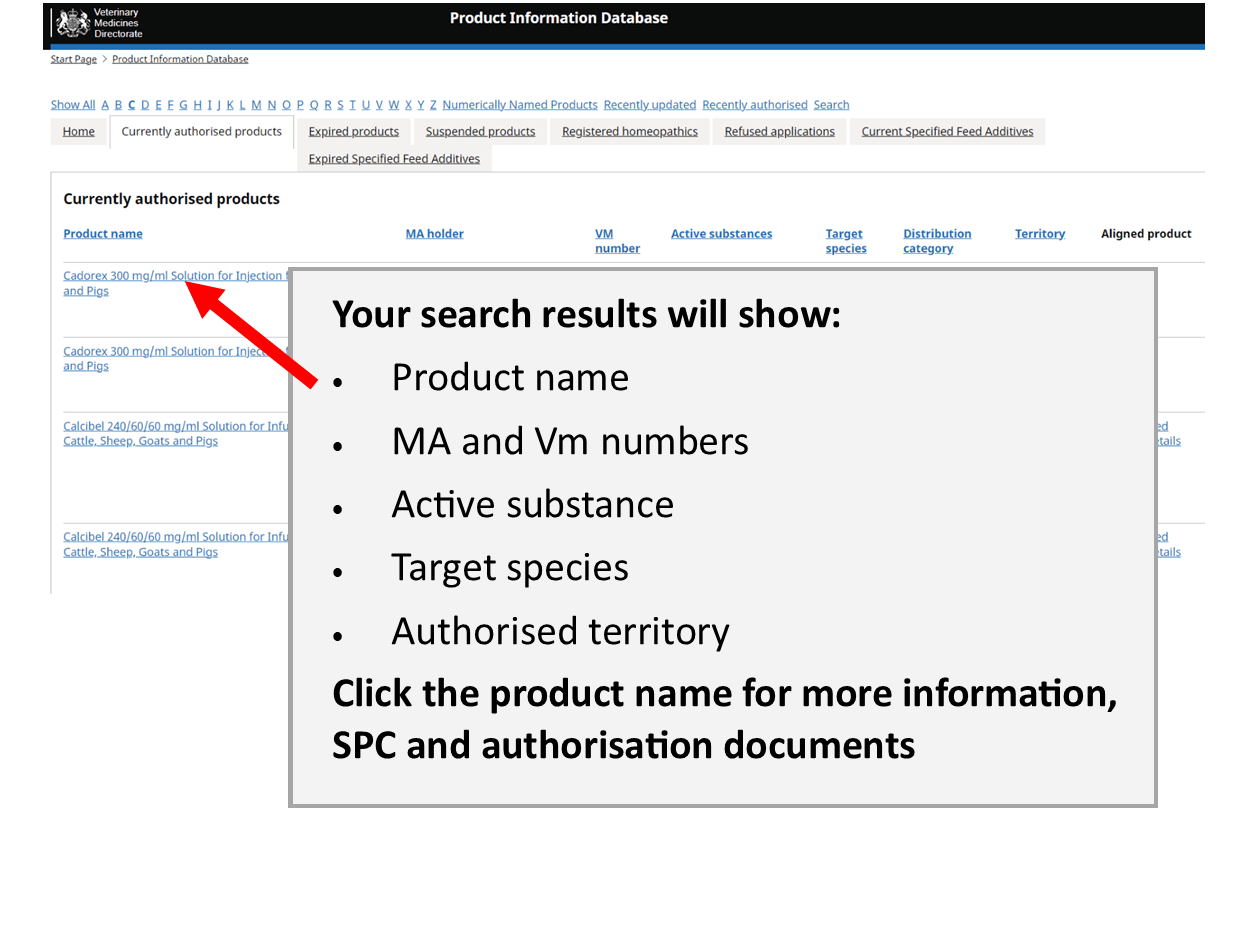
Product Information Database
Our Product Information Database is your primary resource for the most current information about all veterinary medicines authorised in Great Britain and Northern Ireland.
As product details change we immediately update the Summary of Product Characteristics and label text here. However, it may take up to 12 months for changes to appear on marketed packaging.
Products are authorised separately in Great Britain and Northern Ireland. Authorisations may be aligned if they are the same product held by the same authorisation holder.
Product supply and the Special Import Scheme
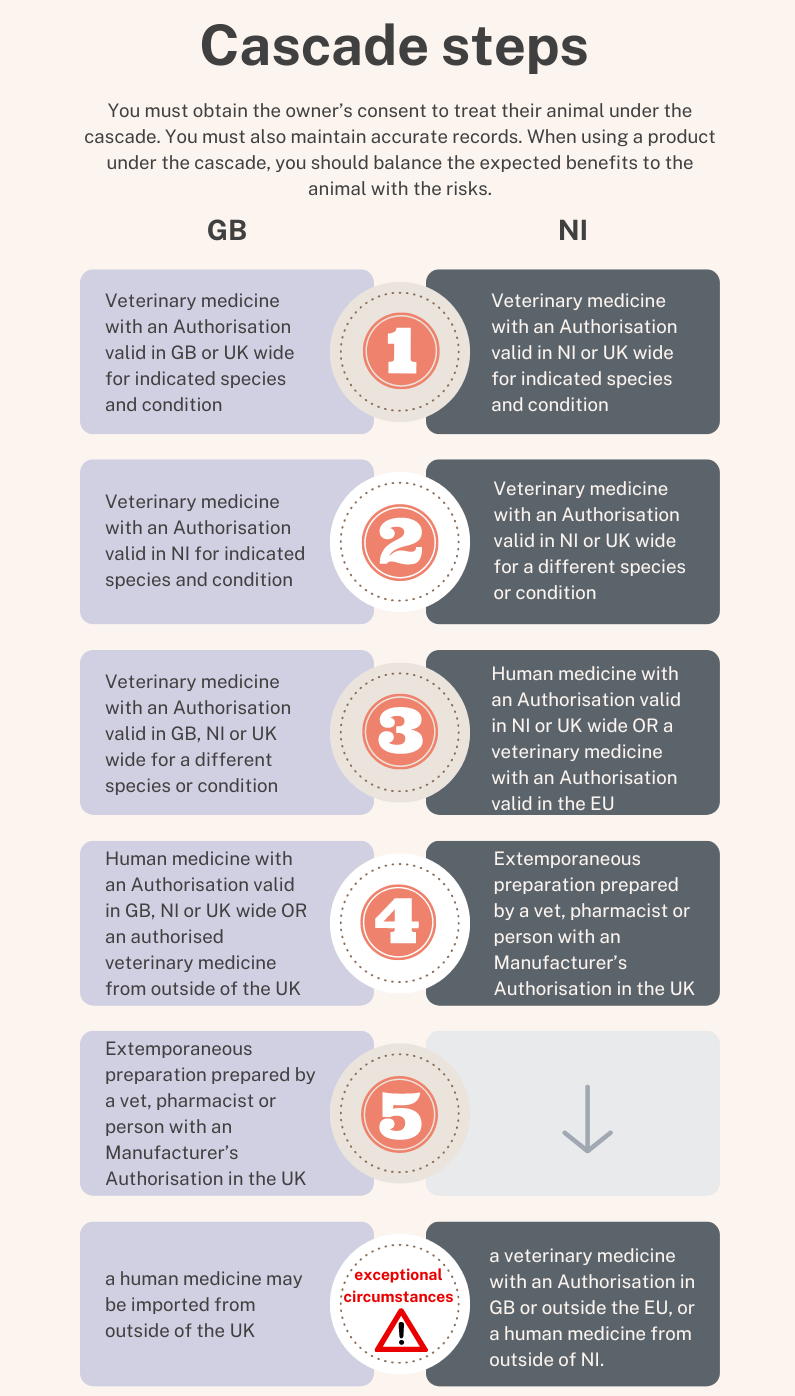
If a suitable veterinary or human medicine authorised in your territory (GB or NI) is not available then vets can use the prescribing cascade to source medicines authorised elsewhere in the world. This is importing, and you cannot do this without a certificate from the VMD.
Learn more about:
- authorisation territories
- the prescribing cascade
- importing and the Special Import Scheme
- FAQs on the Special Import Scheme
- helping us to develop a new import service

Pharmaceuticals in the Environment cross-government group
The Pharmaceuticals in the Environment (PiE) Group is a UK cross-government platform to enable discussion and knowledge exchange relating to PiE from human, veterinary and, where there is cross over, agricultural and non-agricultural sources.
At our first meeting, held in April 2023, we agreed our purpose – to develop a co-ordinated strategy to reduce the impacts of pharmaceuticals on the environment (biodiversity) and human health. We also identified our first priority area, which is to gain a better understanding of the potential sources of fipronil and imidacloprid levels detected in UK surface waters and to work towards reducing these levels.

Adverse events and pharmacovigilance
Vets are one the of the most at risk users of veterinary medicines, particularly from needlestick injuries. Vets are also likely to witness or be informed of adverse events occurring in animals or their owners.
Veterinary staff can provide the Marketing Authorisation Holder with crucial clinical information when reporting adverse events and this contributes to pharmacovigilance processes to ensure the safety and efficacy of veterinary medicines.

Enforcement
We meet our aims through proportionate risk-based regulation and enforcement. Our primary approach is through education to ensure those we regulate have the information they need.
We do take robust action when needed, which can result in prosecution for the most serious cases. We receive information (unverified and unevaluated data) and intelligence (evaluated data) from a range of sources such as the pharmaceutical industry, veterinarians, general public and other enforcement partners, such as the Police and Border Force.
Find out how prescribers can help educate their clients about prescription misuse and fraud.

VMD Training Centre
We offer training opportunities and work closely with other organisations to create educational content about a range of topics.
Find out more about our courses for veterinary dispensary managers and for wholesale qualified persons. We have collaborated with the RCVS to offer a FREE course on managing veterinary medicines.

Equine Anthelmintic Resistance
Anthelmintic resistant parasites are present on most - if not all - equine yards, and anthelmintic resistance is becoming more widespread and of greater significance.
We are actively involved in promoting responsible use. Find out more about how we are working with the equine industry to promote a consistent approach to parasite control.
Also, find out about the pan-industry group CANTER - Controlling ANTiparasitic resistance in Equines Responsibly - and the many organisations contributing to its development.

Share information with your practice and clients
Here is a collection of downloads or links to useful information - share these today!
This site is in BETA - your feedback will help us improve it.