Product supply and the Special Import Scheme
Medicines availability is key to protecting animal health and welfare
Making products available in the UK
Access the medicines you need
Authorisation territories
Products are authorised separately for Great Britain and Northern Ireland. Those authorised before 2021 may continue to hold a UK wide authorisation for both GB and NI.
You can only use products authorised in your territory but if there is no suitable authorised product you can use the Cascade to help source the right medicines.
Our Product Information Database tells you everything you need to know.
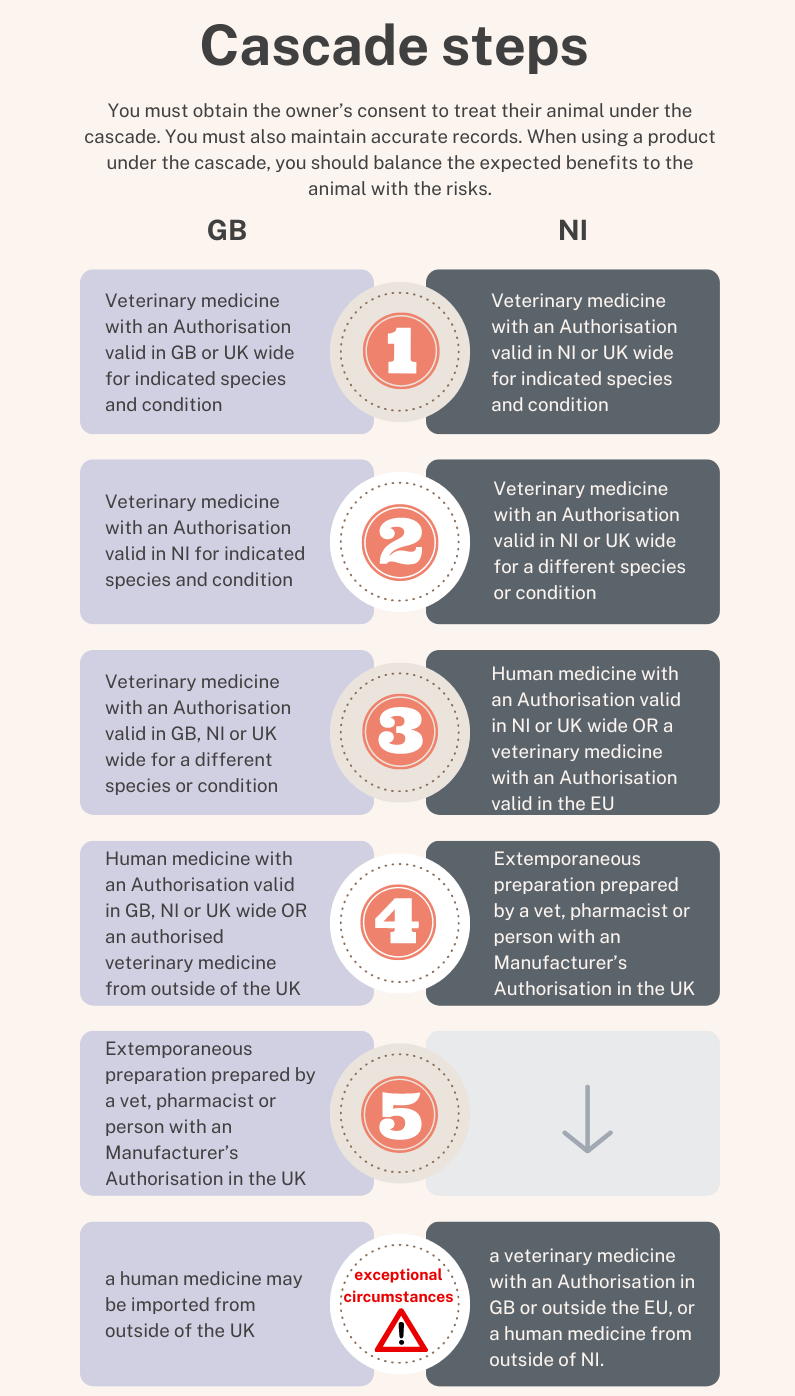
The prescribing cascade
Where there is no suitable veterinary medicine authorised in your territory for the specific condition in the animal being treated, to avoid unacceptable suffering, you can use your clinical judgement to treat animals under your care in accordance with the prescribing cascade.
This is a risk-based decision tree which applies differently in GB and NI. Use the one for your territory to make prescribing decisions on a case-by-case basis.
Before making your decision read:
The Cascade is set out in sequential order of suitability and you should consider the steps in order. It is your responsibility to determine the product that is most suitable for use in the individual animals, which may be based on product characteristics (such as pharmaceutical form or strength), prior evidence or experience of use and/or availability of medicines.
There are special considerations for food-producing animals detailed in our guidance.
Importing a medicine
Should you need to use a product not authorised in your territory, such as importing veterinary medicines authorised in GB to NI or from outside the UK, you will need to apply for a Special Import Certificate. You must check with suppliers that it is available before you apply, and you must have your certificate before you buy.
Importing controlled drugs
Products which fall within the scope of the Misuse of Drugs Regulations 2001 may need an additional approval from the Home Office.
Special Import Scheme
Once you have considered the Cascade and decided you need to source a product not authorised in your territory, even if your supplier is based in the UK, you will need an import certificate from the VMD. This includes sourcing medicines from NI which are not authorised in GB, and vice versa.
List of Services
- Registering with us
List Item 1
Only vets registered with the RCVS can apply for a Special Import Certificate. You will need an account before you can apply which can take up to 24 hours to activate - so register now to avoid future delays.
- Checking with suppliers
List Item 2
Most regulatory agencies around the world have product databases like ours. This will help you identify what veterinary medicines are authorised in their country. You should contact a supplier before you apply to make sure the product you need is available.
- Applying online
It is easy to apply online and certificates for products that have previously been imported may be available instantly. For others, you must provide as much information as possible in your application.
**New Import Service now available**
Did you know....
Vm numbers tell you where a product is authorised
- if the Vm number begins with XXXXX/5XXX then it is authorised for veterinary use in Great Britain
- if it begins with XXXXX/3XXX or EU/X/XX/XXX/XXX then it is authorised for use in Northern Ireland
- if it begins with XXXXX/4XXX then its authorised in both GB and NI
If a product doesn't have a Vm number then its not authorised in the UK
They may be authorised in another country, be authorised for human use (PL number), or specially compounded/manufactured for a specific animal and use. These products are unauthorised, and only a veterinary surgeon can prescribe these under the Cascade on a case-by-case basis after a clinical assessment of the animal under their care.
Products prescribed under the cascade cannot be advertised or promoted
This is because they do not hold a veterinary marketing authorisation in the UK, and therefore have not been subject to a full assessment of quality, safety and efficacy. Veterinary surgeons should only proceed with treatment when satisfied that a positive benefit:risk assessment has been reached for the animal(s) under their care.
This site is in BETA - your feedback will help us improve it.
